Abstract
Introduction
Omidubicel is an advanced cell therapy for allogeneic hematopoietic stem cell transplantation (HSCT), derived from appropriately HLA-matched umbilical cord blood (UCB) and comprised of ex-vivo expanded CD133+ cells and a non-cultured lymphocyte-containing fraction. Recent results of a global phase III trial of omidubicel vs standard UCB showed more rapid hematopoietic recovery, reduced rates of infection, and shorter hospitalizations in patients (pts) randomized to omidubicel (Horwitz et al, Blood, 2021). We now report results of correlative immune reconstitution (IR) studies in this trial (NCT02730299).
Methods
A total of 125 pts aged 13-65 with hematologic malignancies were randomized to allogeneic transplantation with omidubicel or standard UCB following myeloablative conditioning; 108 pts were transplanted per protocol. An optional IR sub-study was conducted and blood was collected at intervals from Day 7 through one year post-transplant. Cryopreserved samples were analyzed in a central laboratory (Covance) using 16-color and 14-color panels and flow cytometric assays to explore T cell, NK cell, B cell, monocyte, and dendritic cell (DC) subsets. Means, medians, ranges, and standard errors were used to summarize cell counts, and one-tailed t-tests were used to compare counts in the two treatment arms.
Results
A total of 37 pts from 15 sites consented to the IR sub-study, representing 34% of the per protocol population; 17 pts were transplanted with omidubicel and 20 pts with control (15 [75%] of control with double UCB). Median age was 30 (range: 13-62) years for omidubicel pts and 43 (range: 19-55) years for controls in the sub-study. Median CD3+ content of omidubicel prior to cryopreservation was lower (180 x 10^6; range: 71-580 cells) than that of controls post-thaw (516 x 10^6, range: 183-990 cells).
Omidubicel pts achieved neutrophil engraftment at a median of 10 (range: 6-28) days post-transplant compared to a median of 18.5 (range: 14-40) days in controls. Omidubicel pts had fewer BMT-CTN Grade 3 viral infections in the first-year post-transplant than controls (6% vs. 25%, respectively).
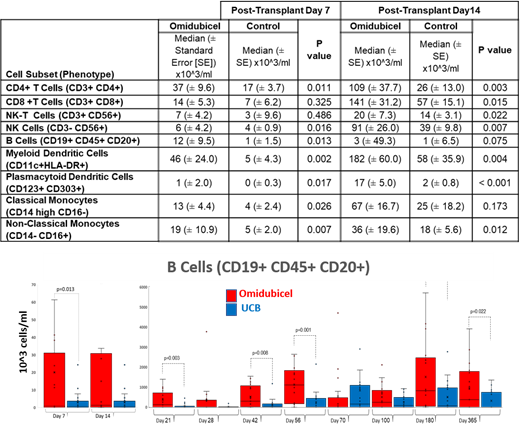
At Day 7 post-transplant, CD4+ T cell counts were significantly higher in omidubicel pts (37x10^3 cells/ml) than in controls (17x10^3 cells/ml, p=0.011). B cells (12x10^3 vs 1x10^3 cells/ml, p=0.013) and NK cells (6x10^3 vs 3 x10^3 cells/ml, p=0.016), as well as monocyte and DC subsets, were also significantly higher in omidubicel pts (Table). Day 14 results similarly demonstrated higher counts of circulating immune cell subsets in omidubicel pts than in controls (Table). Higher B cell counts were observed in omidubicel pts than in controls at 6 months ([863±463] x10^3 vs. [543±221] x10^3 cells/ml, p=0.03) and one year ([1492±370] x10^3 vs [763±150] x10^3, p=0.02) following transplant (Figure).
Conclusions
Circulating immune cell subsets were consistently higher in omidubicel pts than controls as early as one week after transplant, and higher B cell counts persisted through one year. These findings correlated with the clinical observation of fewer severe bacterial, fungal, and viral infections in pts treated with omidubicel compared to standard UCB. These results demonstrate that rapid hematopoietic recovery in pts transplanted with omidubicel is accompanied by the early and robust appearance of a broad array of lymphocyte, monocyte, DC, and NK cell subsets, despite substantially fewer numbers of these cells infused, suggesting a facilitator effect of omidubicel on their in vivo expansion.
Szabolcs: Gamida Cell: Consultancy; Prevail Therapeutics: Consultancy; Sotiria/Forge Biologics: Current equity holder in publicly-traded company. Levy: Gamida Cell: Current Employment. Yackoubov: Gamida Cell: Current Employment. Pato: Gamida Cell: Current Employment. Galamidi-Cohen: Gamida Cell, Ltd: Current Employment. Horwitz: Gamida Cell: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal